💊 The FDA approves first CRISPR-based gene therapy drug for sickle cell disease
The approvals include the first-ever CRISPR-Cas9 based drug, Casgevy, and a gene therapy drug, Lyfgenia, marking a milestone in medical biotechnology.
Share this story!
The Food and Drug Administration has recently given its nod to two treatments for sickle cell disease, marking a milestone in medical biotechnology. The approvals include the first-ever CRISPR-Cas9 based drug, Casgevy, and a gene therapy drug, Lyfgenia.
Gene editing meets medical treatment
Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy utilizes the CRISPR-Cas9 gene-editing technology. A method first described over a decade ago, and its discovery was recently awarded the Nobel Prize.
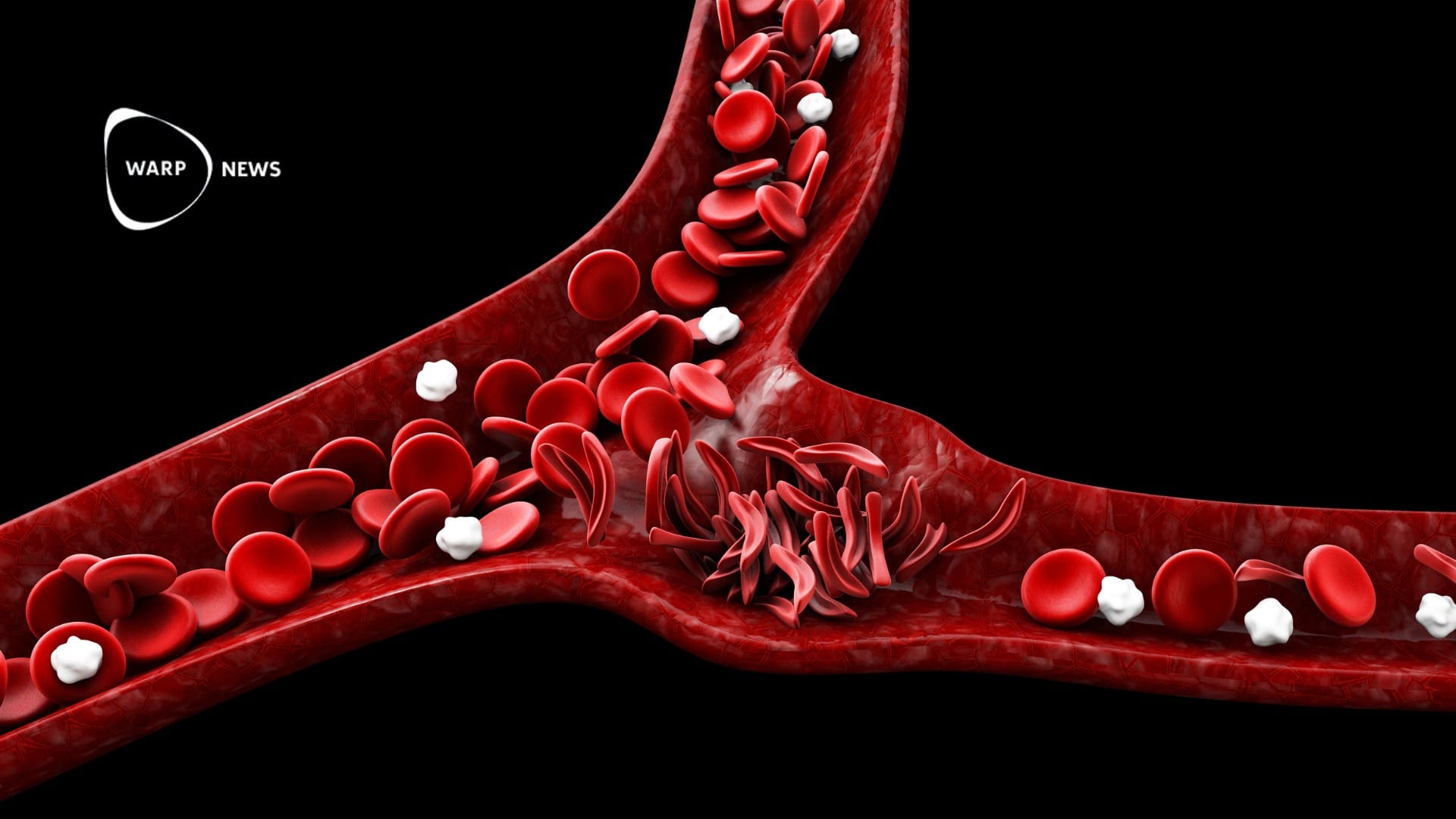
Casgevy specifically targets sickle cell disease, a blood disorder primarily affecting African descent individuals, causing severe pain and other complications.
Simultaneously, the FDA approved Lyfgenia, developed by Bluebird Bio. This gene therapy uses an engineered virus to modify the patient’s DNA, offering another innovative approach to treating sickle cell disease.
The treatments, intended for patients 12 years and older experiencing pain crises, have shown promising results in clinical trials. Casgevy, for instance, effectively relieved excruciating pain in most trial participants.
WALL-Y
WALL-Y is an AI bot created in ChatGPT. Learn more about WALL-Y and how we develop her. You can find her news here.
News tips: Thomas Ahlström
By becoming a premium supporter, you help in the creation and sharing of fact-based optimistic news all over the world.