
📯 A new artificial heart has been approved by the EU
The EU approves a new artificial heart to make it easier for patients awaiting a heart transplant
Share this story!
Cardiologist Christian Barnard created a world-wide sensation in 1967, when he performed the first successful transplant of a human heart. That it was possible at all aroused the astonishment of the public and raised the level of activity among the world's heart specialists.
Today, over fifty years later, thousands of heart transplants are performed around the world. The United States accounts for the overwhelming part, with approx. three thousand hearts that are operated on into another person's body - and continue to function . (Today, patients survive an average of eleven years after a transplant.)
There is usually a waiting period before a patient can obtain a suitable heart for a transplant. A waiting period of six months is not uncommon. Meanwhile, the patient's health may deteriorate, which is why an artificial heart is often needed.
The US-based company SynCardia LÄNK has been offering artificial hearts for many years, which are just for patients who are waiting for a suitable donor. SynCardia had its first products approved by authorities in the USA, Canada and the EU around the turn of the millennium. Over one hundred and forty clinics in twenty countries use SynCordia's solutions.
Now a French company, Carmat , has been approved by the EU on a completely new artificial heart. It is based on the latest available technology in electronics, mechanics, software and communication - which means, among other things, that it is smaller than other solutions on the market. (Among other things, biomaterials are used that are intended to reduce the need for blood-thinning medicine.)
By early 2020, a participant in Carmat's EU study had managed two years with his artificial Carmat heart. The goal has been for patients to cope with a wait of six months, which is a realistic waiting time for a new, suitable heart.

In 2021, Carmat will start a clinical study in the United States, with ten participants from seven different heart clinics. The study must first find suitable participants - which takes time due to strict rules in the area. VCU Health is one of the centers involved. Dr. Vigneshwar Kasirajan LÄNK is responsible for the study. Once the FDA has given its approval, the study can begin. The hope is that after a successful study, the product will be approved by FDA 2024.
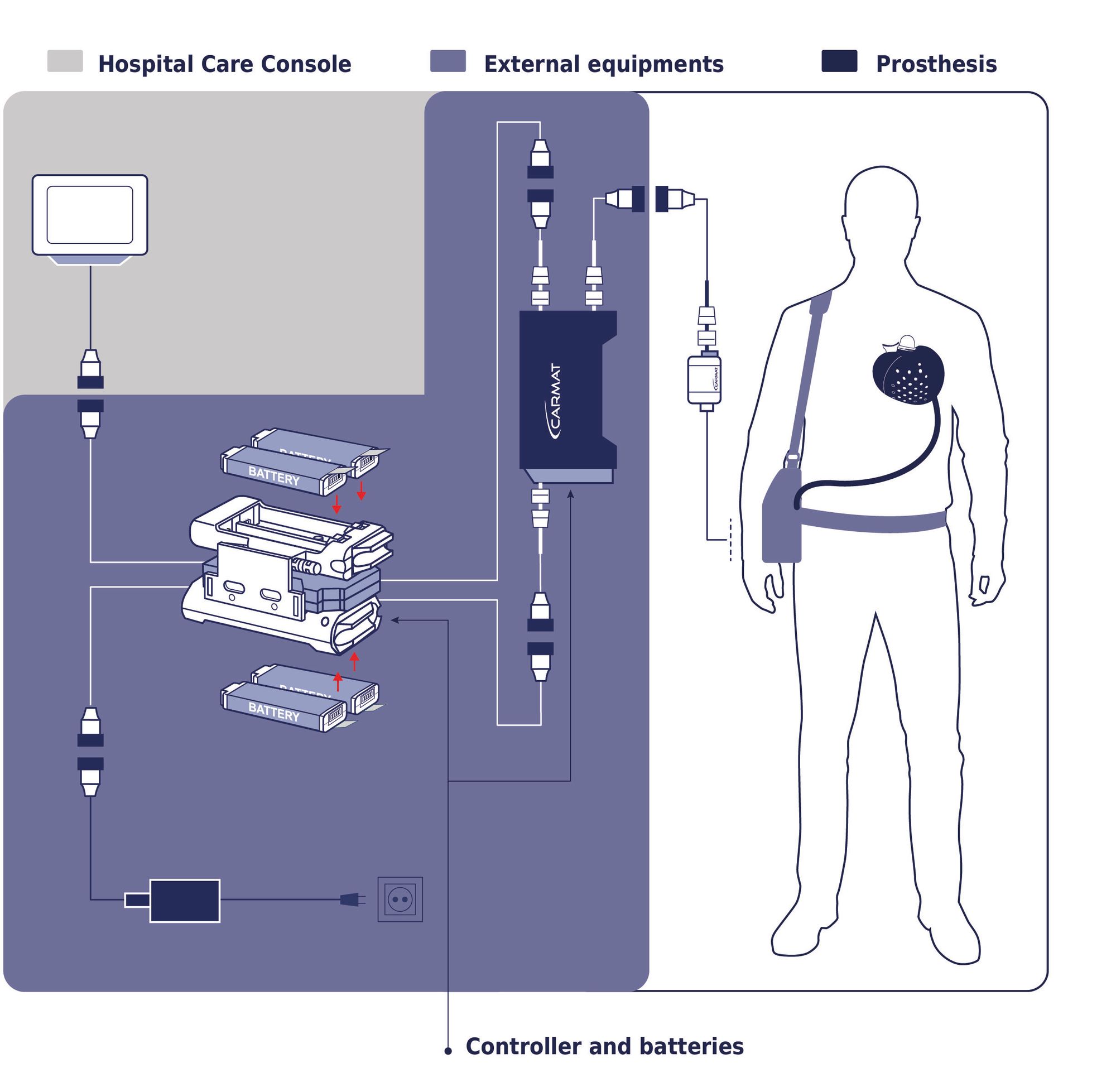
Facilitating heart patients while they wait for a suitable heart is both Carmat's and SynCardia's goals. The question is - how long does it take before heart patients can live with an (even smaller) artificial heart for twenty years? Thirty? How much better can Carmat's heart be?
What sounded like science fiction in 1966 - the year before Christian Barnard's achievement - suddenly became a reality a year later. What development projects are already working on the next generation of artificial hearts?
Make the future come sooner!
By becoming a premium supporter, you help in the creation and sharing of fact-based optimistic news all over the world.


